 StatCan COVID-19: Colorectal cancer screening during the COVID-19 pandemic: Impact of paused screening and evaluation of strategies to reduce delays
StatCan COVID-19: Colorectal cancer screening during the COVID-19 pandemic: Impact of paused screening and evaluation of strategies to reduce delays
Archived Content
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please "contact us" to request a format other than those available.
by Jean H. Yong and Rochelle E. Garner
Skip to text
Acknowledgements
The authors would like to thank the members of the COVID-19 and Cancer Global Modelling Consortium’s colorectal cancer working group, who contributed to the study design, methods, data and results interpretation. The authors would also like to acknowledge the members of the National Colorectal Cancer Screening Network and the Screening Resilience and COVID-19 Working Group, who identified pan-Canadian priorities for colorectal screening resilience and provided thoughtful feedback on this analysis. In addition, the authors would like to thank Claude Nadeau for help with the analysis, as well as the individuals who provided thoughtful feedback on this article: Erika Nicholson, David Armstrong, Heather Bryant and Scott Antle. Finally, this analysis was conducted using OncoSim—a tool and program led and supported by the Canadian Partnership Against Cancer, with model development by Statistics Canada and funding from Health Canada. The list of individuals who made significant contributions to the development of OncoSim is available at www.OncoSim.ca.
Colorectal cancer is projected to be the third-most-diagnosed cancer and the second leading cause of cancer-related death in Canada in 2020 (Brenner et al. 2020). Early detection of colorectal cancers and detection of treatable pre-cancerous polyps through preventive screening have been proven to save lives and are cost-effective (Coldman et al. 2015; Heitman et al. 2010; Telford et al. 2010). Individuals can access cancer screening through either participating in organized screening programs or seeing a primary care provider (i.e., opportunistic screening). Beginning in 2007, organized colorectal screening programs were established in Canada and are now in place in most provinces and in one territory (Canadian Partnership Against Cancer 2018). Colorectal cancer screening for individuals at average risk uses a self-collected fecal immunochemical test (FIT). The results of the FITs are quantitative (numerical), and the screening programs set a threshold (cut-off value) for abnormal tests, above which the individual is referred for a colonoscopy to identify whether the blood is due to cancer, an advanced adenoma, or some other cause. The follow-up colonoscopy also allows for the removal of adenomas, with the goal of preventing colorectal cancer.
Colorectal cancer screening, along with other health care services, was suspended in Canada in the initial phase of the COVID-19 pandemic response. This pause was deemed necessary to allow health care facilities to establish appropriate infection-control measures to prevent COVID-19 outbreaks and to reserve health system capacity for COVID-19 patients. Access to colonoscopies was also reduced in many settings due to concerns of COVID-19 infection with aerosolized procedures. Organized colorectal cancer screening programs have mostly resumed, as has some opportunistic screening through individuals’ health care providers.
Earlier work has examined the potential impact of paused cancer screening programs on future cancer incidence and mortality (Yong et al. 2020). The current article projects the impact of a three-month suspension of screening for colorectal cancer using a fecal test for average-risk individuals, and compares strategies to minimize the harm from screening interruptions. The projections come from OncoSim, a cancer microsimulation model co-developed by Statistics Canada and the Canadian Partnership Against Cancer (Gauvreau et al. 2017).
Impact of colorectal cancer screening interruptions
If no screening interruptions had occurred, the simulation estimated that approximately 540,000 individuals in Canada would have undergone colorectal cancer screening using a fecal test between April 1 and June 30, 2020. If these individuals are not invited for screening until they are due two years later (i.e., no catch-up screening), these 540,000 missed screenings could lead to approximately 10,000 people with adenomas and colorectal cancers going undetected in 2020. This could result in nearly 440 colorectal cancer deaths in the long term.
Strategies to mitigate the risks from colorectal cancer screening interruptions
To mitigate the risks posed by screening interruptions, the relevant programs would need to invite individuals who would have normally undergone screening during the interruptions to undergo screening when service resumes (i.e., catch-up screening). However, inviting all individuals to be screened at the same time when service resumes would overwhelm the colonoscopy capacity allocated for the follow-up of an abnormal FIT. This would be in addition to the colonoscopy backlog resulting from the suspension of colonoscopy services for non-screening-related indications that occurred during the initial pandemic phase. Two potential strategies could help reduce the surge in colonoscopy demand from catch-up screening: (1) reduce the fecal-test screening backlog over a longer period, rather than inviting all individuals at the same time; and (2) increase the fecal-test threshold that is used to refer individuals for a follow-up colonoscopy.
OncoSim was used to explore the potential impact of different strategies to clear the screening backlog in an effort to mitigate the risks from interruptions to colorectal cancer screening. The strategies considered three recovery periods (or intervention durations)—6, 12 and 24 months—and various FIT thresholds that could reduce the number of individuals sent for a follow-up colonoscopy. These scenarios were explored as part of an international comparative modelling project with the COVID-19 Global Modelling Consortium (www.ccgmc.org). When trying to reduce the screening backlog over a longer period, it was assumed that programs would delay ongoing screening slightly (e.g., six weeks later than their scheduled screening, on average), allowing programs to prioritize the backlogged screeners. During the recovery period, when programs are trying to reduce the screening backlog, programs could refer individuals for a colonoscopy follow-up by either using the usual FIT threshold or setting a higher threshold to reduce the number of individuals sent for a follow-up colonoscopy.
OncoSim projections showed that, if programs could restrict the ongoing screening delay to a shorter period (e.g., six weeks), catch-up screening could avert most of the excess cancer deaths attributable to screening interruptions (Chart 1). Using the regular FIT threshold, programs would require scheduling 50%, 25% or 4% more FIT-triggered colonoscopies than usual over the course of the catch-up period, if they were to catch up with the fecal-test screening backlog over 6, 12 or 24 months, respectively (Chart 1).
Many programs are currently operating at approximately 90% of their usual colonoscopy capacity because of COVID-19-related constraints. If programs had access to only 90% of their usual capacity for FIT follow-up colonoscopy procedures, temporarily increasing the FIT threshold by 25% (i.e., from 20 to 25 µg Hb/g faeces) for 24 months would reduce the number of expected excess deaths by 20% (i.e., 90 deaths).
To minimize the potential for colorectal cancer deaths from paused cancer screening, OncoSim scenarios showed that increasing the fecal test follow-up colonoscopies by 4% for the next 24 months: this could reduce the number of deaths due to paused screening by 93% (i.e., 409 deaths). Without increasing the total number of colonoscopies, additional follow-up colonoscopies could be done if fewer colonoscopies were conducted for lower-yield indications, such as primary colonoscopy screening or surveillance colonoscopy for patients who were previously diagnosed with low-risk adenomas. Depending on jurisdictional constraints and population needs, varying the FIT threshold, increasing the availability of colonoscopy resources, or reducing the catch-up period could reduce the expected number of excess colorectal cancer deaths.
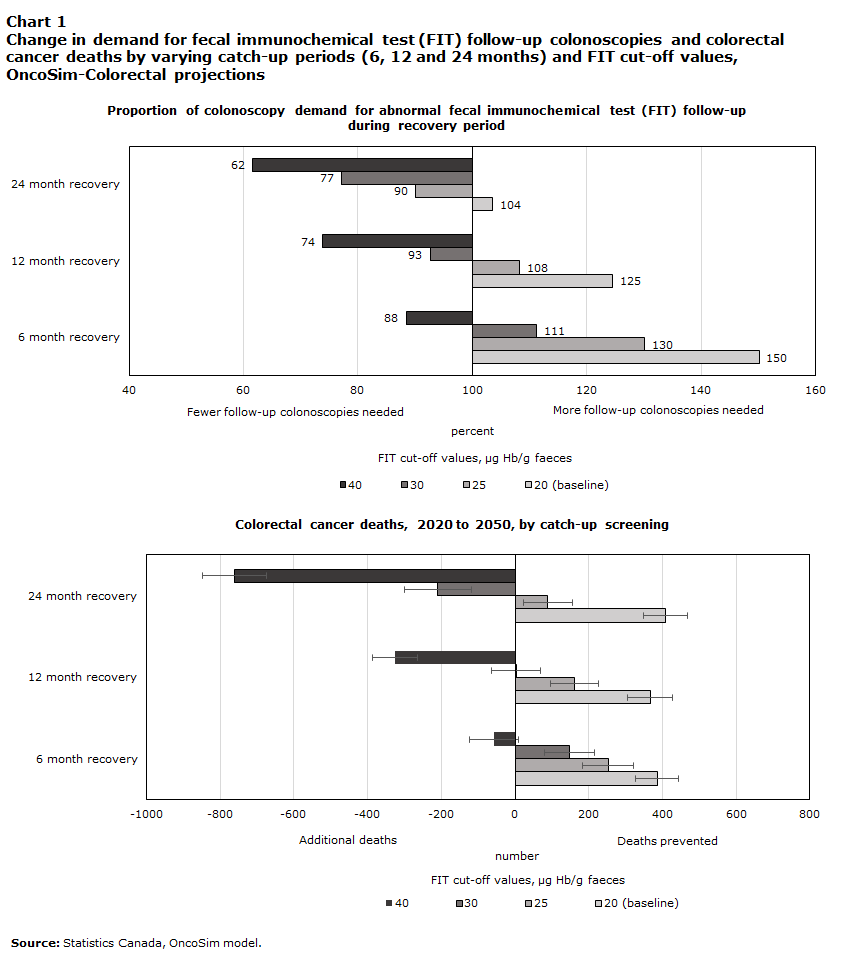
Data table for Chart 1
| FIT cut-off values, µg Hb/g faeces | ||||
|---|---|---|---|---|
| 20 (baseline) | 25 | 30 | 40 | |
| percent | ||||
| Proportion of colonoscopy demand for abnormal fecal immunochemical test (FIT) follow-up during recovery period | ||||
| 24 month recovery | 104 | 90 | 77 | 62 |
| 12 month recovery | 125 | 108 | 93 | 74 |
| 6 month recovery | 150 | 130 | 111 | 88 |
| number | ||||
| Colorectal cancer deaths, 2020 to 2050, by catch-up screening | ||||
| 24 month recovery | 409 | 89 | -210 | -762 |
| 12 month recovery | 366 | 162 | 3 | -326 |
| 6 month recovery | 385 | 253 | 148 | -57 |
| Source: Statistics Canada, OncoSim model. | ||||
Conclusion
Colorectal cancer screening interruptions that result from the COVID-19 pandemic response could lead to an increase in cancer deaths. Catch-up screening can mitigate most of the risks associated with these interruptions, but would require additional colonoscopy capacity to ensure follow-up for abnormal FIT results. This highlights the importance of evaluating alternate strategies to reallocate colonoscopy resources, such as using different FIT thresholds for determining follow-up or increasing the volume of colonoscopies for a specified period of time. Local contextual factors – such as resource availability, COVID-19 infection rates, and colorectal cancer rates – could be considered by programs when determining the optimal approach.
The present study made some simplifying assumptions for modelling purposes, which may not be generalizable to all situations. For example, the OncoSim scenarios assume that 43% of Canadians participate in colorectal cancer screening (see “Data sources and methods” section). However, screening participation rates are known to differ across jurisdictions and by socioeconomic and demographic characteristics (Kiran et al. 2017; Decker et al. 2016; Blair et al. 2019). Decision makers in specific provinces and territories may wish to evaluate the impact of various catch-up scenarios according to the participation rates within their communities. Furthermore, although not examined in the current study, catch-up scenarios that would seek to improve screening participation in under-screened populations or to prioritize higher-risk individuals for screening in the recovery period could be considered in future studies.
In addition, the present study considered the impact of only a single period of paused cancer screening. However, as Canada experiences multiple waves of COVID-19 cases, screening and diagnostic services may be affected on multiple occasions, exacerbating the backlog of missed screenings. The impact of multiple or prolonged screening pauses would be greater than that presented here, and this could also be examined in future studies.
Data sources and methods
Dynamic microsimulation, in the context of social science and population health, is the simulation of large samples of individuals (micro) and their behaviours, states and actions over time (dynamic). OncoSim is an empirically grounded, dynamic microsimulation tool that evaluates cancer-control strategies for prevention, screening and treatment (Statistics Canada 2018). The OncoSim model and program is led and supported by the Canadian Partnership Against Cancer, with funding from Health Canada and model development by Statistics Canada (Gauvreau et al. 2017).
OncoSim–Colorectal simulates the natural history and progression of colorectal adenomas and cancer (Coldman et al. 2015). The model assumes that most colorectal cancers develop from adenomas: in OncoSim, adenomas can progress in size from small (≤5 mm) to medium (6 mm to 9 mm) to large (≥10 mm), can transform into stage I preclinical cancer, or can regress. The model assumes that screening can detect adenomas and colorectal cancers at a preclinical (i.e., no symptoms) stage. Through calibration, the model replicates the prevalence of adenomas from the literature and the colorectal cancer incidence and mortality for the Canadian population from the Canadian Cancer Registry. Stage-specific survival rates were calibrated to match the number of cancer-specific deaths expected from the Canadian Vital Statistics Death Database. The model assumes that screen-detected cancers have better stage-specific cancer survival rates than those that are detected clinically. The impact of increasing the FIT threshold on test performance (sensitivity and specificity) came from a Dutch cohort study (de Klerk et al. 2019).
In OncoSim–Colorectal, it is assumed that organized screening programs that use FIT for average-risk individuals are in place across Canada and have a participation rate of approximately 40%. The model scenarios also assume that the screening participation rate would return to 40% immediately upon the resumption of colorectal cancer screening programs. More details about modelling assumptions are available in an earlier publication (Yong et al. 2020).
References
Blair A., L. Gauvin, S. Ouédraogo, and G.D. Datta. 2019. “Area-level income disparities in colorectal cancer screening in Canada: Evidence to inform future surveillance.” Current Oncology 26 (2): e128–e137.
Brenner, D.R., H.K. Weir, A.A. Demers, L.F. Ellison, C. Louzado, A. Shaw, D. Turner, R.R. Woods, L.M. Smith, and the Canadian Cancer Statistics Advisory Committee. 2020. “Projected estimates of cancer in Canada in 2020.” Canadian Medical Association Journal 192: E199–E205.
Canadian Partnership Against Cancer. 2018. Colorectal Cancer Screening in Canada: Environmental Scan. Toronto: Canadian Partnership Against Cancer.
Coldman, A.J., N. Phillips, J. Brisson, W. Flanagan, M. Wolfson, C. Nadeau, N. Fitzgerald, and A.B. Miller. 2015. “Using the cancer risk management model to evaluate colorectal cancer screening options for Canada.” Current Oncology 22: e41–e50.
Decker, K.M., A.A. Demers, Z. Nugent, N. Biswanger, and H. Singh. 2016. “Reducing income-related inequities in colorectal cancer screening: Lessons learned from a retrospective analysis of organised programme and non-programme screening delivery in Winnipeg, Manitoba.” BMJ Open 6: e009470. Available at: doi: 10.1136/bmjopen-2015-009470.
de Klerk, C.M., E. Wieten, I. Lansdorp-Vogelaar, P.M. Bossuyt, M.C. Spaander, and E. Dekker. 2019. “Performance of two faecal immunochemical tests for the detection of advanced neoplasia at different positivity thresholds: A cross-sectional study of the Dutch national colorectal cancer screening programme.” The Lancet Gastroenterology and Hepatology 4: 111–118.
Gauvreau, C.L., N.R. Fitzgerald, S. Memon, W.M. Flanagan, C. Nadeau, K. Asakawa, R. Garner, A.B. Miller, W.K. Evans, C.M. Popadiuk, M. Wolfson, and A.J. Coldman. 2017. “The OncoSim model: Development and use for better decision-making in Canadian cancer control.” Current Oncology 24: 401–406.
Heitman, S.J., R. Hilsden, F. Au, S. Dowden, and B.J. Manns. 2010. “Colorectal cancer screening for average-risk North Americans: An economic evaluation.” PLOS Medicine 7: e1000370.
Kiran, T., R.H. Glazier, R. Moineddin, S. Gu, A.S. Wilton, and L. Paszat. 2017. “The impact of population-based screening program on income- and immigration-related disparities in colorectal cancer screening.” Cancer Epidemiology Biomarkers & Prevention 26 (9): 1401–1410.
Statistics Canada. 2018. “Projecting the future of health in Canada using microsimulation modelling.” StatCan Blog. Available at: https://www.statcan.gc.ca/eng/blog/cs/microsimulation.
Telford J., A.R. Levy, J.C. Sambrook, D. Zou, and R.A. Enns. 2010. “The cost-effectiveness of screening for colorectal cancer.” Canadian Medical Association Journal 182: 1307–1313.
Yong, J.H., J.G. Mainprize, M.J. Yaffe, Y. Ruan, A.E. Poirier, A. Coldman, C. Nadeau, N. Iragorri, R.J. Hilsden, and D.R. Brenner. 2020. “The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada.” Journal of Medical Screening. Available at: doi: 10.1177/0969141320974711. Online ahead of print.
- Date modified:
